What is the resolution to the osmotic doughnut paradox?
We fill a torus shaped circular tube with its central axis parallel to the ground with water to about 80% completion. Then place semi-permeable membranes across the cross section at the top and bottom of the tube. Finally, saturate one side with salt. Since we filled it to 80% there is some air at the top (by the way, poke a hole at the top to remove any explanations of air pressure), but is is conceivable that the osmotic pressure will raise the level on the salty side to completion. Since our ideal membranes have no resistance to water, pure water pours across the top membrane, falls, spins a turbine, and solves the world's energy crisis.
The problem
This is what the apparatus looks like:
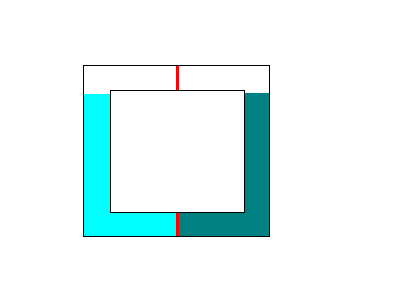
The red lines denote semipermeable membranes. The dark blue liquid on the right is a saturated solution, and the light blue liquid on the left is, say, distilled water.
According to the paradox, water should first move from the left to right through the bottom membrane, along the osmotic gradient. This causes the water level on the right to rise. As Taras says, there is "no resistance" to the flow of water by the top membrane, so when the water level on the right clears the bottom of the top membrane, water flows through the top membrane, back into the left side. This then continues indefinitely since the salt can't move.
The solution
The errorneous assumption is that water must flow through the top membrane because it "offers no resistance" to the flow of water and because there is no water on the left hand side to oppose the flow of water out of the right hand side. This is a faulty way of thinking about osmosis.
The key is that there is a force opposing the flow of water across the membrane. Water molecules on the right experience strong attraction to the solute particles. There is no solute on the left, so the potential energy actually increases when you pull water from the right to the left. You actually need to exert pressure on the right in order to force water to move to the left. It will not occur spontaneously as the question presupposes, so there is no perpetual motion. (As an aside, this process of forcing a solvent through a semipermeable membrane is called reverse osmosis, and is used to purify water.)